The Periodic Table of the Elements was perhaps the first time science found a pattern in nature of the kind so often previously postulated by people proposing mystical schemes for organizing the Universe:
H
* * He
Li Be B C N O F Ne
Na Mg Al Si P S Cl Ar
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mr Ds Rg Cn Nh Fl Mc Lv Ts Og
After Lawrencium, element 103, elements 104 and 105 were called Un-nil-quadium and Un-nil-pentium, but now elements 104 through 109 are officially named Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, and Meitnerium, and apparently the next two elements are known as Darmstadtium and Roentgenium as well.
Since I wrote this, other elements have gained names.
Element 118 is called Oganesson, and element 114 is called Flerovium, for example.
After Roentgenium, the next element, 112, is Cn, Copernicum, and the list continues: Nh, Nihonium; Fl, Flerovium; Mc, Moscovium; Lv, Livermorium; Ts, Tennessine; Og, Oganesson.
The Russian chemist Dimitri Mendeleyev is normally credited with the discovery of the Periodic Table; the German chemist Julius Lothar Meyer may have preceded him slightly, but he met with delays in publication. Like Mendeleyev's version of the Periodic Table, Meyer's version also recognized that the period between elements having corresponding properties increased for heavier elements due to the space taken by the transition metals.
Before these two chemists, an imperfect form of the Periodic Table assuming only one period of recurrence had been proposed by three other scientists: Alexandre E. Béguyer de Chancurtois, in 1862, John A. R. Newlands in 1863, and W. Odling in 1864.
And Leopold Gmelin may have anticipated them all back in 1843; although he merely made a chart of elements with similar properties, and did not claim any periodic law, as he did order them by their atomic weights, his table did at least appear in hindsight to suggest the idea.
There are names for the groups of elements in the Periodic Table:
----- Alkali Metals
/ -- Alkaline Earth Metals
| / --- Coinage Metals
| | / He
Li Be | B C N O F Ne
Na Mg | Al Si P S Cl Ar
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mr Ds Rg Cn Nh Fl Mc Lv Ts Og
| || | | | | |
| || | | | | \
\ / | | | | \ --- Inert Gases, Noble Gases
------------------------- Transition Metals ------------------------- | | | \ ------ Halogens
| | \ --------- Chalcogens
| \ ------------ Pnictogens
\ --------------- Tetrels
------------------ Triels, Boron Group Elements
---- Rare Earths: Lanthanides plus Scandium and Yttrium
/
|- Sc
|- Y
- La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu -- Lanthanides
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr -- Actinides
The lighter halogens are stronger than the heavier ones, whereas the heavier alkali metals are stronger than the lighter ones; that is, they are more reactive.
This is a consequence of a general property of how electronegative and electropositive ions behave chemically, and thus this property tells us about the behavior of elements in other columns of the Periodic Table as well.
Carbon is obviously not a metal; Tin and Lead, on the other hand, have always been recognized as metals, but they're all in the same column in the Periodic Table.
So, while the metals are on the left of the Periodic Table, and the non-metals are on the right, the boundary between them is not vertical but slanted.
Li Be |B ]C N O F Ne Na Mg Al|Si]P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga[Ge|As Se]Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn[Sb|Te]I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi[Po|At]Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mr Ds Rg Cn Nh Fl Mc Lv Ts Og
There is no universally agreed-upon boundary between metals and nonmetals.
One conventional line includes Aluminum, Germanium, Antimony, and Polonium as metals, and Boron, Silicon, Arsenic, Tellurium, and Astatine as non-metals.
Other chemists include a group of semi-metals or metalloids between the metals and non-metals.
Boron, Silicon, and Astatine are sometimes included in this group, and sometimes classed as non-metals.
Germanium, Arsenic, Antimony, Tellurium, and Polonium are usually included on most lists of semi-metals or metalloids.
Since Selenium is shiny and metallic in appearance, I'm somewhat surprised, despite its position in the Periodic Table in the same column as oxygen and sulfur, that it, too, isn't recognized as a semi-metal.
Also, there were different systems of numerical designation for the columns of the Periodic Table:
IA IIA IVA VIA VIII IB IIIB VB VIIB European, former IUPAC
IIIA VA VIIA IIB IVB VIB 0
1A 2A 3A 4A 5A 6A 7A 8... 8 1B 2B 3B 4B 5B 6B 7B 0
IA IIA IVB VIB VIII IB IIIA VA VIIA American
IIIB VB VIIB IIB IVA VIA VIIIA
1A 2A 3B 4B 5B 6B 7B 8... 8 1B 2B 3A 4A 5A 6A 7A 8A
1 2 3... 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Current IUPAC
H He
Li Be B C N O F Ne
Na Mg Al Si P S Cl Ar
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mr Ds Rg Cn Nh Fl Mc Lv Ts Og
In order to let the table fit, I've just used the two-letter symbols for the elements, but of course not all of them will be familiar, so here is a key:
1 H Hydrogen 2 He Helium
3 Li Lithium 4 Be Beryllium 5 B Boron 6 C Carbon 7 N Nitrogen 8 O Oxygen 9 F Fluorine 10 Ne Neon
11 Na Sodium 12 Mg Magnesium 13 Al Aluminum 14 Si Silicon 15 P Phosphorus 16 S Sulfur 17 Cl Chlorine 18 Ar Argon
19 K Potassium 20 Ca Calcium 31 Ga Gallium 32 Ge Germanium 33 As Arsenic 34 Se Selenium 35 Br Bromine 36 Kr Krypton
37 Rb Rubidium 38 Sr Strontium 49 In Indium 50 Sn Tin 51 Sb Antimony 52 Te Tellurium 53 I Iodine 54 Xe Xenon
55 Cs Cesium 56 Ba Barium 81 Tl Thallium 82 Pb Lead 83 Bi Bismuth 84 Po Polonium 85 At Astatine 86 Rn Radon
87 Fr Francium 88 Ra Radium 113 Nh Nihonium 114 Fl Flerovium 115 Mc Moscovium 116 Lv Livermorium 117 Ts Tennessine 118 Og Oganesson
21 Sc Scandium 22 Ti Titanium 23 V Vanadium 24 Cr Chromium 25 Mn Manganese 26 Fe Iron 27 Co Cobalt 28 Ni Nickel 29 Cu Copper 30 Zn Zinc
39 Y Yttrium 40 Zr Zirconium 41 Nb Niobium 42 Mo Molybdenum 43 Tc Technetium 44 Ru Ruthenium 45 Rh Rhodium 46 Pd Palladium 47 Ag Silver 48 Cd Cadmium
57 La Lanthanum 72 Hf Hafnium 73 Ta Tantalum 74 W Tungsten 75 Re Rhenium 76 Os Osmium 77 Ir Iridium 78 Pt Platinum 79 Au Gold 80 Hg Mercury
89 Ac Actinium 104 Rf Rutherfordium 105 Db Dubnium 106 Sg Seaborgium 107 Bh Bohrium 108 Hs Hassium 109 Mr Meitnerium 110 Ds Darmstadtium 111 Rg Roentgenium 112 Cn Copernicum
58 Ce Cerium 59 Pr Praesodymium 60 Nd Neodymium 61 Pm Promethium 62 Sm Samarium 63 Eu Europium 64 Gd Gadolinium 65 Tb Terbium 66 Dy Dysprosium 67 Ho Holmium 68 Er Erbium 69 Tm Thulium 70 Yb Ytterbium 71 Lu Lutetium
90 Th Thorium 91 Pa Protactinium 92 U Uranium 93 Np Neptunium 94 Pu Plutonium 95 Am Americum 96 Cm Curium 97 Bk Berkelium 98 Cf Californium 99 Es Einsteinium 100 Fm Fermium 101 Md Mendelevium 102 No Nobelium 103 Lw Lawrencium
Later on, particle physics found a symmetry in the properties of heavy short-lived particles that eventually was explained in terms of the quark model...

and the arrangement of ten items in a triangle was considered to be the holy Tetractys by the Pythagoreans. Another famous set of ways to link ten items together is discussed in the page entitled A Peculiar Deck of Cards
The binary number sequence, now so frequently encountered in connection with computers, also forms the basis of the I Ching or Book of Changes (perhaps more literally, the Change Classic). The order in which the hexagrams are discussed in the I Ching is not the numerical binary order, but the following:
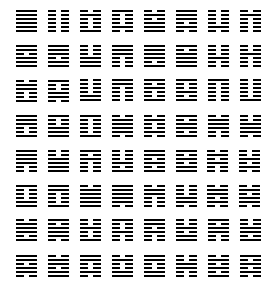
While no simple mathematical rule has been found to account for this complete sequence, it certainly does have many symmetries.
The hexagrams in this sequence are clearly grouped into 32 pairs. In each pair, the second hexagram is usually the result of reversing the sequence of solid and broken lines in the first one; only if the sequence of the first one would be the same when reversed is the second one, instead, inverted from the first one by replacing solid lines with broken lines, and broken lines with solid ones.
The first two hexagrams are all solid lines and all broken lines, and the last two both consist of alternating solid and broken lines.
A Western form of divination based on binary sequences is known as Geomancy; not the Feng Shui of China, but rather the Ilm al-Raml of the Middle East.
Copyright (c) 2005 John J. G. Savard